Are you ready to find 'how to write formula for precipitate'? Here you can find questions and answers on the topic.
Composition Precipitation EquationsDetermine the formulas for the possible products victimisation the general bivalent displacement equation. ...Predict whether either of the possible products is water hopeless. If either executable product is hopeless, a precipitation chemical reaction takes place, and you will extend with step 3. ...Follow these stairs to write the complete equation.
Table of contents
- How to write formula for precipitate in 2021
- Formula of the precipitate
- Precipitation reaction examples
- How to make a precipitate
- How is a precipitate formed
- Precipitation reaction produce which salt
- Formula of precipitate calculator
- Write a balanced equation for the precipitation reaction
How to write formula for precipitate in 2021
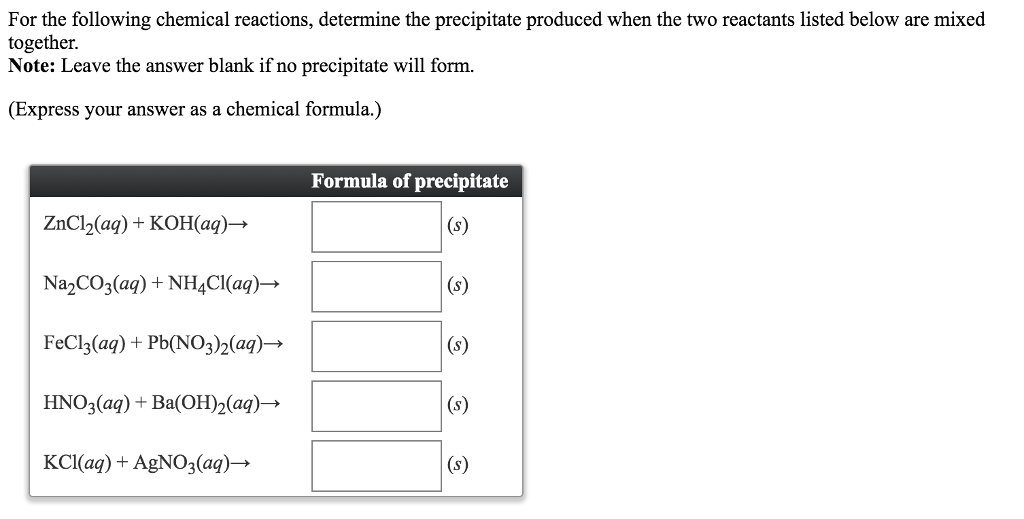 This picture demonstrates how to write formula for precipitate.
This picture demonstrates how to write formula for precipitate.
Formula of the precipitate
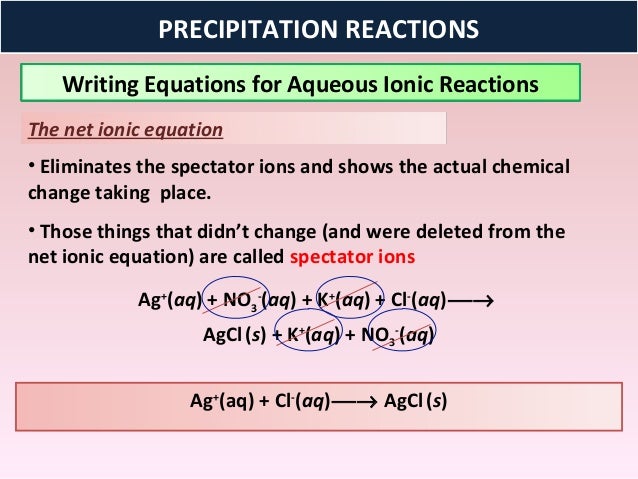 This image shows Formula of the precipitate.
This image shows Formula of the precipitate.
Precipitation reaction examples
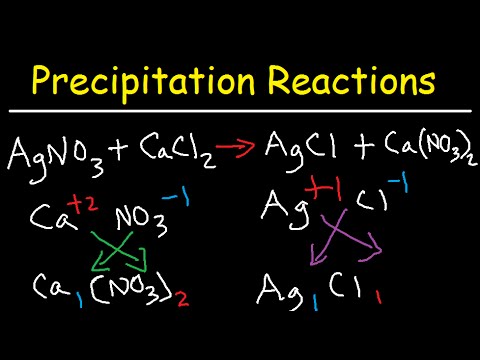 This picture representes Precipitation reaction examples.
This picture representes Precipitation reaction examples.
How to make a precipitate
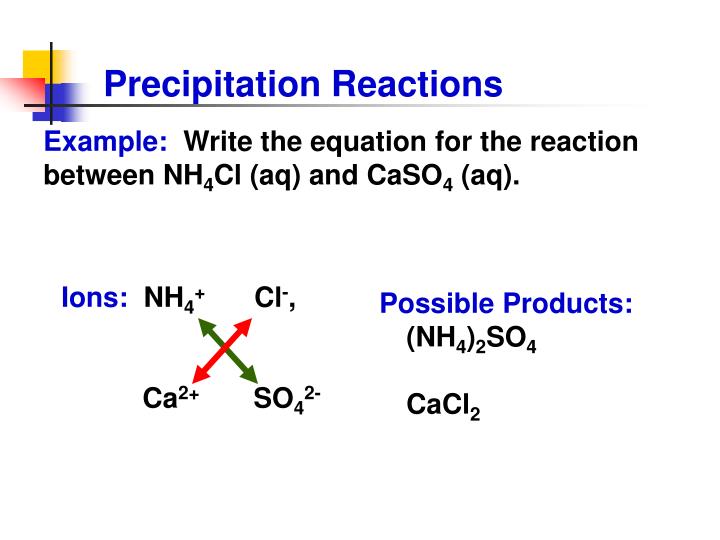
 This picture shows How to make a precipitate.
This picture shows How to make a precipitate.
How is a precipitate formed
 This image demonstrates How is a precipitate formed.
This image demonstrates How is a precipitate formed.
Precipitation reaction produce which salt
 This picture representes Precipitation reaction produce which salt.
This picture representes Precipitation reaction produce which salt.
Formula of precipitate calculator
 This image representes Formula of precipitate calculator.
This image representes Formula of precipitate calculator.
Write a balanced equation for the precipitation reaction
 This picture demonstrates Write a balanced equation for the precipitation reaction.
This picture demonstrates Write a balanced equation for the precipitation reaction.
How do you write the complete ionic equation?
Write the complete ionic equation by describing water-soluble ionic compounds as separate ions and insoluble ionic compounds with a complete formula. Eliminate the formulas for the ions that are unchanged in the reaction (the spectator ions).
How to write the complete equation for precipitation?
Step 3: Follow these steps to write the complete equation. Write the formulas for the reactants separated by a “+”. Separate the formulas for the reactants and products with a single arrow. Write the formulas for the products separated by a “+”. Write the physical state for each formula. 1) The insoluble product will be followed by (s).
How are nitrate and sodium ions eliminated in a precipitation equation?
The nitrate and sodium ions have the same form on each side of the equation, so they are eliminated as spectator ions. EXAMPLE 2 – Predicting Precipitation Reactions: Predict whether a precipitate will form when water solutions of barium chloride, BaCl2(aq), and sodium sulfate, Na2SO4(aq), are mixed.
How are solubility rules used in a precipitation reaction?
Solubility Rules can be used to predict if a product will be insoluble (forms a precipitate) 2 in aqueous solutions at 25°C. A precipitation reaction is a chemical reaction which produces a precipitate when solutions are mixed together. Spectator ions are ions in solution that are not used to form the precipitate.
Last Update: Oct 2021
Leave a reply
Comments
Bonna
27.10.2021 04:21Typically you will glucinium asked to far dissect a chemic equation by authorship not only the molecular equation, only additionally the self-contained ionic and ultimate ionic equations.